- Elena González Lab
Understanding human cells is our passion, specifically we are interested in cell identity and cell potential, properties that have been shown to be extremely plastic depending on cell environment.
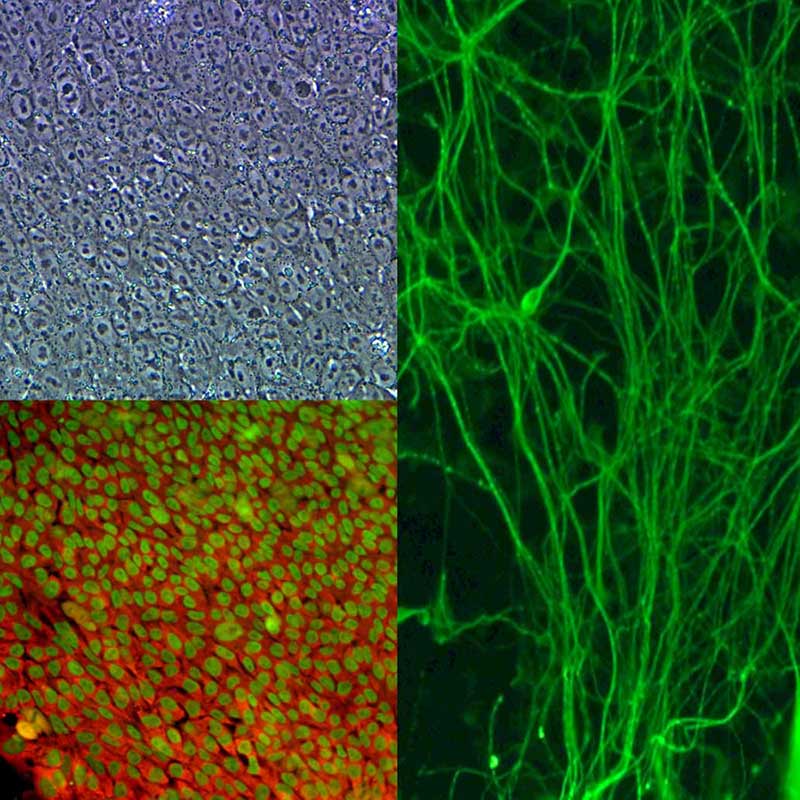

Cellular reprogramming can be briefly defined as the transformation of a specialized cell into a pluripotent cell, a cell that can self-renew by dividing and also differentiate into all cells of the adult body, but not extra-embryonic tissues such as the placenta.
When this transformation is caused by the over expression of specific transcription factors, typically the OSKM combination – OCT4, SOX2, KLF4 and c-Myc – described by Shinya Yamanaka in 2006, the cells generated are called induced pluripotent cells (iPSCs).
Although theoretically this is a “simple” protocol, cellular reprogramming is still a very inefficient process and more importantly, the molecular mechanisms governing the transformation of a somatic cell into an iPSC are still not completely understood.
The oocyte is definitively the most powerful cell in terms of “natural” reprogramming, and so we use the oocyte as source of information to study human pluripotency acquisition.


Our long-term goal is to provide new insights into cellular reprogramming by analyzing molecular pathways, and identifying new factors that could play an important role in cell reprogramming.
The results of our work contribute to the understanding of human pluripotency and of -otherwise inaccessible – human development, and also to inform the development of safe and efficient cell reprogramming protocols to be tested in pre-clinical and clinical models of human disease.
Cell reprogramming is also used in our laboratory for neurodegenerative disease modeling. Whether or not a disease can be treated often depends on whether we can gain a good understanding of its basic biology. Disease modelling using iPSC technology allows scientists to explore how a disease works in the laboratory, to search affected pathways and alternative targets for potential treatments.
